The FDA approved Xolair (Omalizumab) as a food allergy treatment! This is amazing news for our food allergy community. Many thanks to the researchers and clinical trial families who participated in this trial, you are heroes!
I am so pleased to have the opportunity to interview Dr. Ahmar Iqbal, MD, MBA, Genentech’s Executive Medical Director of Respiratory and he is also an OUtMATCH study co-author of the NEJM manuscript.
Disclaimer: This interview is for informational purposes only and should not be considered medical advice. Please contact your medical care team regarding any health or allergy related questions.
FDA Approves Xolair as a Food Allergy Treatment
On February 16, 2024 the FDA made an announcement that they “approved Xolair (omalizumab) injection for immunoglobulin E-mediated food allergy in certain adults and children 1 year or older for the reduction of allergic reactions (Type I), including reducing the risk of anaphylaxis, that may occur with accidental exposure to one or more foods.”
Then on February 25, 2024, The New England Journal of Medicine published the article “Omalizumab for the Treatment of Multiple Food Allergies” which describes the OUtMATCH trial and results in greater detail. This particular phase of the trial mainly focuses in on the effectiveness of using Xolair (omalizumab) alone as a monotherapy to increase one’s food allergy tolerance. According to the NIH, Xolair “can help protect kids with multiple food allergies during accidental exposure.”
180 people were randomized into either a treatment or placebo group and received injections for 16-20 weeks either once or twice a month. “A total of 79 of the 118 participants (67%) receiving omalizumab met the primary end-point criteria, as compared with 4 of the 59 participants (7%) receiving placebo (P<0.001). Results for the key secondary end points were consistent with those of the primary end point (cashew, 41% vs. 3%; milk, 66% vs. 10%; egg, 67% vs. 0%; P<0.001 for all comparisons).” You can read about the details of the trial in the NEJM article.
The results from phase 1 of this study seem to indicate that the shots alone may help people with multiple food allergies tolerate accidentally eating small amounts of their allergen.

Q&A with Dr. Ahmar Iqbal, M.D., M.B.A.
When I was asked whether I would like to interview someone about this trial, of course I said yes!! I’ve been following the Xolair clinical trials for 10+ years. Our family had to decide whether we wanted to participate in a food allergy clinical trial at Stanford. I understand some of the concerns and hope you will find this Q&A interview helpful. I had the pleasure of speaking with Dr. Iqbal during our call. I have edited our question and answer interview for brevity and clarity.
What is Xolair (Omalizumab) and what can we learn from this phase of the trial as a therapy for food allergies? How does it compare with other food allergy treatments?
Dr. Iqbal: The only other FDA approved treatment is called Palforzia. It is a medicine with a small amount of peanut flour in it and what you do is you take it daily so that your body becomes used to the peanut flour, so that the body recognizes peanuts as not something bad and not react to it anymore. You have gradually up the dose (over time) and consume a dose every day. The treatment is only for peanut.
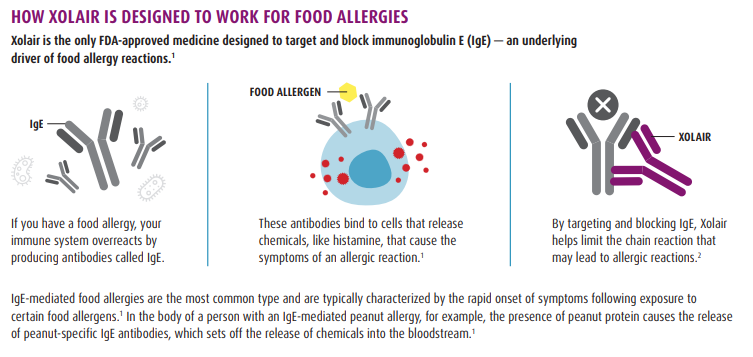
Our body is supposed to recognize food and not react to it when we eat. For someone with a food allergy, they have antibodies called IgE which binds to immune cells called mast cells and basophil cells and signals the immune cells to release antihistamines and other chemicals which lead to an allergic reaction.
Xolair is an anti-IgE that binds to IgE cells, which then blocks the IgE from binding to mast cells and basophil cells (to prevent them from releasing histamines) and hopefully suppresses a severe allergic reaction and potentially serious allergic reactions or other medical complications.
The primary endpoint was for peanut but four other foods were studied. Everyone had to have a peanut allergy plus two other foods that they were allergic to and these foods were supposed to be representative of the roughly 160+ foods that people could be allergic to.
What is the typical patient experience with Xolair treatment?
Dr. Iqbal: Xolair is a subcutaneous injection that is given right under the skin. Subcutaneous injections are less uncomfortable than intramuscular injections. The dosing is based upon the patient’s total IgE and weight. Whether the patient is a child or an adult, the doses could be it could be given once a month. If larger doses are needed, it would have to be given twice a month.
Patients will need to receive the shots at a doctor’s office to monitor in case of any reactions. After the first few doses at the doctor’s office to stay and observe any reactions. If the patients are ready, doctors may recommend and train patients or caregivers to give the shots at home.
How do patients typically respond to the Xolair treatment?
Dr. Iqbal: Xolair has been around about 20 years for different indications such as asthma, nasal polyps, and hives (also known as chronic spontaneous urticaria). It’s generally very well tolerated and as you can imagine we have a lot of safety data on it.
But for this food allergy study, injections were given over 16 to 20 weeks. Then some patients, 60 of them, received what’s called open label. The patient knows that they’re receiving the drug and is not blinded anymore. The first phase of the trial is a randomized control trial meaning that you don’t know whether you are in the treatment group or placebo.
How do you know if you’re feeling better with the injections? When you have a food allergy and accidentally eat your food allergen you might have an allergic reaction. With Xolair, you might accidentally eat your food allergen and hopefully the reaction will be mild or not happen. This doesn’t mean that patients can be complacent. You still have to avoid the foods that you’re allergic to and carry your epinephrine.
How do patients and allergists decide when to adjust the frequency of Xolair shots or discontinue?
Dr. Iqbal: There are three stages of this ongoing trial and long-term studies are still needed to answer this question fully. When patients start to use Xolair, they don’t want to let go of the protection this treatment offers so they may need to continue taking Xolair. Their doctor can decide what to do with some tests, such as allergen specific IgE blood tests to gauge progress and to determine how to help patients stay protected with ongoing treatment and for how long.
Just like for other conditions such as asthma, patients taking Xolair for their food allergies need to take their medication for a long time. Their doctor can decide based on a patient’s clinical condition. Because the exposure to food allergens can happen anytime and you’re taking Xolair to prevent or reduce reactions to allergen exposure, it is not recommended at this time to discontinue the treatment.
How do allergists determine who might be a good candidate for Xolair treatment? What do patients need to consider?
Dr. Iqbal: During the trial, we also make sure that each of these patients have a confirmed full allergy. We measured the specific IgE for their allergens and administered skin prick tests.
In clinical practice, physicians take a detailed history and they they will ask the parent if the patient is a child very carefully what happened, when was the food consumed, and what exactly happened. If people have had moderate to severe allergic reaction, then that’s when Xolair is indicated.
So you wouldn’t do it if you ate an allergenic food and it just caused a little mild tingling in your mouth or a little hive around your mouth, those are considered mild. There’s a grading scale that allergists use and they apply it to find out if you have you had in the past a moderate to severe allergic reaction to indicate when Xolair might be helpful.
A reader whose child tried OIT but had to discontinue due to stomach issues is wondering whether treating with Xolair only might help? What are some considerations they might discuss with their doctor in this scenario?
Dr. Iqbal: I would recommend that patients to have a conversation with their allergist and look at the stage 2 and stage 3 results when published to guide decisions. We hope we will have more answers in the fall.
I would recommend very strongly that you talk with your allergist, that you heard about Xolair and what’s the best option for that individual patient. I think there should be a dialogue between the physcian, family, and patient. Allergists are very well trained and I’m happy to say a lot of them turned up at our AAAAI session as the data was presented.
There’s a black box warning about anaphylaxis; what are some precautions to consider regarding potential allergic reaction to Xolair? What are some potential side effects of Xolair?
Dr. Iqbal: The black box warning has been there not for food allergy but from the other indications (asthma, nasal polyps, etc.). In this case if you look at the label that the FDA has provided, it is there to make sure people understand that Xolair is a drug that can prevent anaphylaxis and still be aware that in case of anaphylaxis, they should be using epinephrine right away.
The drug label says that Xolair is supposed to reduce the possibility of anaphylaxis. But the black box warning is there so that people are especially aware that anaphylaxis can happen with food or many drugs. It’s important to discuss any concerns with your doctor about any risk, which depends on your history, what you are allergic to, and how frequently you have reactions.
It’s recommended to have the first three or four injections in the doctor’s office so they can monitor for any reactions from the drug itself. Keep in mind that not many drugs are free from any kind of risk, but the incidence is 0.2%, so it’s a relatively lower risk. You have to consider the benefits of the treatment compared to the risks. In this study, the patients didn’t experience severe reactions to Xolair. The only two reactions we observed in this study were injection site reaction and fever. That is actually very promising since we had nearly 200 patients who received Xolair in this study.
How much will the treatment cost? Have you heard about any insurance companies covering this treatment for food allergies yet, or is it still too early to know?
Dr. Iqbal: I’m just a medical expert and will have someone from our team provide some information for your readers. Xolair has been around for nearly 20 years to treat other conditions with good coverage. We are optimistic that using Xolair to treat food allergies will be similarly covered as well.
How is Xolair a treatment for food allergies and not a cure? Are patients still considered allergic, and do they need to continue carrying epinephrine auto-injectors?
Dr. Iqbal: Patients taking Xolair for food allergies still need to carry epinephrine autoinjectors because we cannot predict very well who will have a severe reaction. Anyone can experience anaphylaxis and it can be provoked by many things, even exercise.
There are two important points we need to remember. First is that epinephrine is a life saver. The need for epinephrine will be lower but you cannot take the risk of not carrying epinephrine. Second, Patients still need to avoid their allergens.
I wish to emphasize that if someone has a severe allergic reaction, it’s an emergency. You’ve got to use epinephrine right away. Xolair is a long-term therapy, and it will not help in an emergency situation when you need cardiovascular system supported with epinephrine, an emergency treatment. Xolair is a maintenance treatment that hopefully will decrease the need for emergency treatment.
Do you have any encouragement for anyone who might feel apprehensive about the treatment?
Dr. Iqbal: First of all it’s natural to be afraid of taking an injection every month or every two weeks for a long time. So it’s absolutely normal and natural for people to be apprehensive. And that’s a good thing in the sense that then you are aware of what the potential side effects of the drug are and then have a good discussion with your doctor to be more informed.
Your doctor should be able to listen to your concerns and help you make the best decision based on your test results and clinical history. You can make these decisions together with the doctor whether the benefits outweigh the risks.
In some cases, the concerns are not clinical. We’ve heard very frequently that parents are very worried about when their kids go off to college, when there is peer pressure, or the lack of availability of resources and non-allergenic food in the cafeteria and things like that. They want to make sure their children are protected. I’m just making up a hypothetical patient, but those are situations we hear about and those are good topics to discuss with your allergist.
Similarly, if there’s a younger child, one or two years old, starting daycare or preschool, then they have a greater potential of exposure to their allergens via other people or food and experience an allergic reaction. In some cases, children might undergo learning at home and their exposure risks are different. Those are individual concerns that people should bring up and discuss with their allergist.
If doctors wish to offer Xolair as a food allergy treatment option for their patients, are there educational materials available for doctors to share with patients who might be interested in more information about this treatment?
Dr. Iqbal: The Xolair insert has detailed information for patients about the drug itself, how it should be administered, and what side effects to look for. It’s very clear and we will have other resources about Xolair which the doctors can provide, or your readers can contact us. We are happy to share those informational materials. We want every patient to be super educated about all aspects of Xolair and the condition that they have so that they can manage themselves. We try to provide patients with literature that are developed and approved by the FDA.
Can you give us a preview of what we will learn from the next phase of the outmatch trial?
Dr. Iqbal: I can’t comment too much since the study is still on-going. Your readers can look at our study listing on ClincialTrials.Gov in the meantime, until the rest of the study results are shared later this year.
There’s there’s always lots of questions and we’ll look forward to another update soon. Thank you so much Dr. Iqbal and good luck to you and the rest of the study team.
Dr. Iqbal: Well, first of all thank you so much for taking the time and taking an interest. And it’s always a pleasure to talk to people who like you are so knowledgeable and informed. And just to reiterate, this trial is really unique that it was done by the experts in the field, done as a collaboration with the NIH and the Consortium for Food Allergy. This was a very high quality study and it’s indicated for anyone one years of age and over. The two adverse events in numbers higher than placebo were injection site reaction and pyrexia. Hopefully your audience will appreciate all of this information that we have discussed today.

Related articles:
I had forgotten that I wrote about my son’s experiences with Xolair in 2017. You can read about it in my write up about what happened during 2017. It’s a long post so you’ll need to scroll down to find the section.
Thanks for reading, please help Nut Free Wok!
If you like this post or recipe, please be sure to give a 5 star rating, leave a comment, and share this post! Your support means a lot to me.
Subscribe to Nut Free Wok’s email subscription (be sure to respond to the confirmation email). You will be notified by email next time I publish another post or recipe and I won’t send you spam or share your email address with anyone.
Disclosure/Disclaimer:
I may mention the names of stores and/or brand names of products that I use because readers ask and I share products and sources which I use and think may be helpful to readers, all opinions are my own. Please note that manufacturing practices and ingredients can change at anytime without notice and readers are always responsible for assuring allergen safety before buying or consuming foods. NutFreeWok.com is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to Amazon.com. Thank you for reading!





